Materials Modeling – Molecular to Micro-scale Interactions
The goal of this portion of the research is to model the dynamic interplay of chemical and physical changes in material properties over long time scales. Credit: Image by Joen Hermans (Metal soaps in oil paint: structure, mechanisms and dynamics, PhD thesis, University of Amsterdam, 2017).
Expertise and Overall Approach
Changes that occur within paint layers over time are the result of a complex series of reactions involving a large number of species, resulting in the evolution of volatile compounds, incorporation of oxygen, formation of networks, and alterations in properties. A dynamic and quantitative model of the chemical and structural changes will be useful for the conservation science community to make predictions (a forward model) about how environmental conditions and treatment interventions may impact works of art on time scales not easily observed in the laboratory and for the broader materials community to allow us to understand the complex interplay of chemical and physical changes to materials over long time scales. To this end, a multiscale modeling approach incorporating a range of methods will be utilized, including density functional theory (Miliani and Fantacci, Italy), coarse grained molecular dynamics and random graph modeling (Iedema, Netherlands), and kinetic Monte Carlo calculations (Broadbelt, US).
Topic 1: Microscopic Reaction Mechanisms and Network Topology
Northwestern Student: Rebecca Harmon, Chemical and Biological Engineering
Primary Advisors: Linda Broadbelt (NU), Piet Iedema (U. Amsterdam)
Key Outcome: Real-time, experimental data on the mechanical response of cured and aged oil-based paints that will underpin the model presented in Topic 1.
To capture the changes in chemical composition of the film that occur during oxidative curing and aging of a paint layer, we are investigating the underlying chemistry at the mechanistic level. In addition to using quantum chemical calculations to verify the elementary step-nature of these various competing hypotheses, kinetic parameters are calculated based on transition state theory. The approach within this project encompasses both deterministic (Iedema – Amsterdam) and kinetic Monte Carlo (Broadbelt – Northwestern) models. The kinetic Monte Carlo models have the ability to track large numbers of chemical species, so that the chemical identity and local network topology of the collection of molecules can be followed, including the inhomogeneity of the network. There is, however a computational penalty to be paid for accounting for this level of molecular detail, and the Monte Carlo techniques are not well suited for extension to fully cured states where the polymer network is highly crosslinked. This regime is more appropriately investigated with deterministic models. The aim within this topic is to use the kinetic Monte Carlo formalism to establish realistic compositional and kinetic parameters that can be used as input to the more coarse-grained modeling approaches to network structure and formation developed by the Dutch group.
Current Focus: Current thrust has been focusing on the expansion of the microkinetic model of how oil-based systems cure. Using automated reaction network generation, a foundational model with ethyl linoleate captures oligomer distribution, volatile production, and oxygen uptake, but it lacks in chemistry for minor products such as epoxides that are entirely consumed over the first 100 days of drying. One of the main objectives has been to test plausible reactions from literature that would explain the observed epoxide trends. This project started with a proof of concept using the model developed by collaborators at the University of Amsterdam, and Rebecca continued the work on her model at Northwestern while providing updates to her collaborators.
From investigating the reaction networks, a second objective was understanding what conclusions can be drawn from visualizing the network and probing network topology. This is useful when discussing model reduction and how to maintain a representative model with a few species as possible to improve the computational expense.
A third objective is to test the model with a new model monomer, ethyl linolenate. This monomer is triply unsaturated and allows for cyclization of the fatty acid tail, a complexity lacking in the ethyl linoleate model. A long-term objective is to be able to incorporate data science methods as a tool for managing the complexity of how oil paint dries and ages. In particular, incorporate machine learning in a sparse-data system to develop a prediction tool for end-users like art conservators to use with accelerated aging of paint samples.
Figure 1 below is an example of a generated reaction network when uncatalyzed hydrolysis is selected in implementing the kinetic model of ethyl linoleate. It was found that hydrolysis contributes to the consumption of epoxides without the need for acids to catalyze the reaction. Ongoing work investigates the impact of other epoxide ring-opening reactions to the reaction network. In addition to looking at concentration profiles from the reaction network, the impact of termination criteria on the reaction network topology is also under study. As demonstrated with Figure 1, there is a significant dependence of reaction network size to termination criteria; however, model reduction using rate-based criteria maintains network topology. Future work will visualize not just the connectivity between chemical species but their flux through the network.
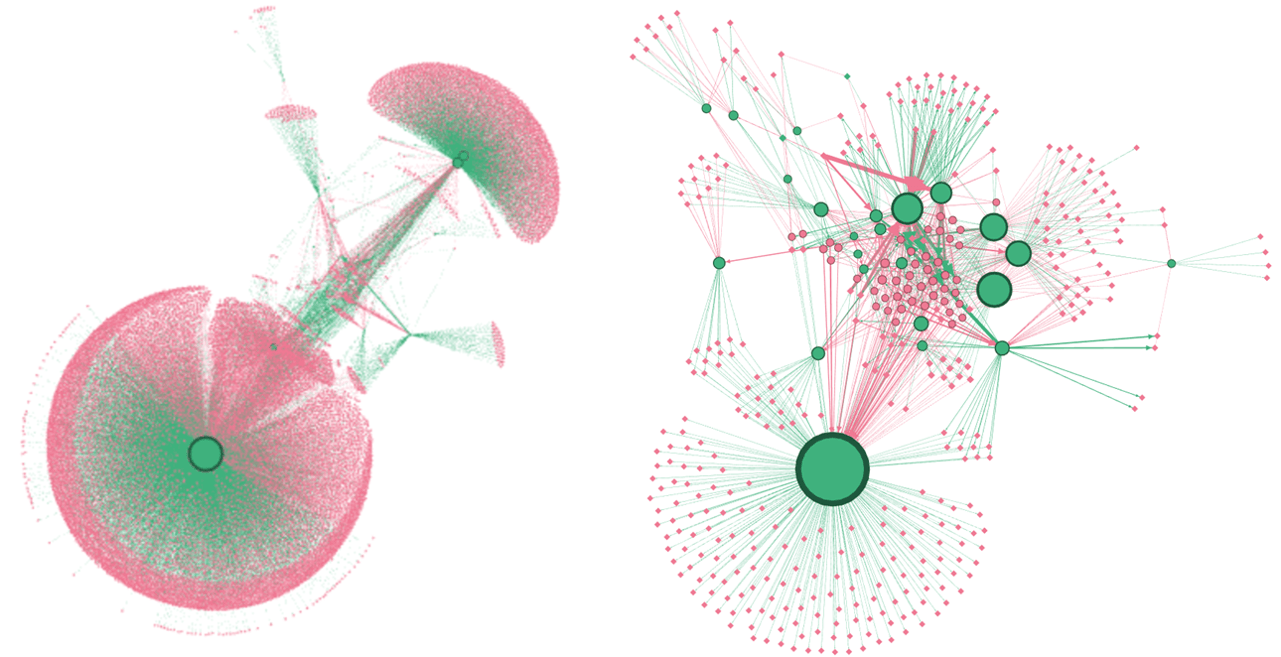 Figure 1: A visualization of unique chemical species (pink) and the reaction families (green) they participate in and the comparison between reaction model termination criteria. Left uses carbon and rank-based criteria with combinatorics to generate products; Right uses species rate-based criteria to generate a pool of only significant species based on a concentration flux threshold. Model reduction using rate-based criteria maintains network topology.
Figure 1: A visualization of unique chemical species (pink) and the reaction families (green) they participate in and the comparison between reaction model termination criteria. Left uses carbon and rank-based criteria with combinatorics to generate products; Right uses species rate-based criteria to generate a pool of only significant species based on a concentration flux threshold. Model reduction using rate-based criteria maintains network topology.
Topic 2: Evolution of Mechanical Response in Oil-Based Paints
Northwestern Student: Gwen DePolo, Materials Science and Engineering
Primary Advisors: Ken Shull (NU), Piet Iedema (U. Amsterdam)
Key Outcome: Real-time, quantitative data on the mechanical response of cured and aged oil-based paints.
In this topic, we use the rheometric quartz crystal microbalance (QCM) to measure both the mass and mechanical response of paint layers in real time. The technique provides both mass and mechanical response of the material at a fixed frequency of 15 MHz. Our focus is on the use of natural triglyceride oils like linseed oil, providing information that enables the material model describe in Topic 1 to be refined. By measuring the temperature dependence of the property evolution, including the mass change, for example, it is possible to extract effective activation energies that can be used to refine the model. Similarly, additional information can be obtained by measuring amount and composition of the soluble fraction obtained by immersing the aged films in an appropriate solvent. These measured quantities can be compared to predictions, adjusting model parameters with the inverse modeling scheme in order to arrive at a refined model. In an effort that is closely coupled with Topic 3 we will investigate the role of metal ions in the mechanical response of the paint. Metal species are present in oil based paints because of their intentional addition as drying agents that catalyze the cure process, or as a biproduct of reactions of the binding medium with inorganic pigments such as zinc oxide.
Current Focus: A current objective of this project has been to develop suitable model thin films that can be analyzed by this technique. A second objective which has been developed is to analyze the most important reactions involved in oil-paint drying mechanisms using polymers more suited to QCM analysis.
For the model linseed oil films used in our in-situ measurements of oil curing and aging with the quartz crystal microbalance, the ideal film thickness is about 1 micron. Stable films in this thickness range are difficult to achieve due to the viscosity of the oil and the tendency for linseed oil to not wet the gold electrode surface of the QCM. One solution is to modify the surface of the quartz crystal using an ultrathin film of polystyrene (~10 nm) to stabilize the oil. While thin films are necessary for analyzing the fully cured films, the early stages of the curing process can be investigated with bulk solutions, with represented data shown in Figure 2. These data are necessary for making a connection to the kinetic modeling part of the PIRE effort described in Task 1.
A second approach for understanding the contributing reactions for film curing and metal soap formation is to probe similar reactions in model systems more suited for QCM and observing general trends which can be correlated with phenomena seen in paints. An example of such a system is using a partially converted poly(tert-butyl methacrylate) (PtBMA) to observe how the hydrolysis reaction of carboxylic acid side chains is affected by the presence of metal cations. The reaction scheme is shown in Figure 3. The results of this experiment will indicate if the pigments in paint, which are often metal cations, causes a faster rate of hydrolysis, releasing more fatty acids into the paint network, which would encourage the formation of metal soaps.
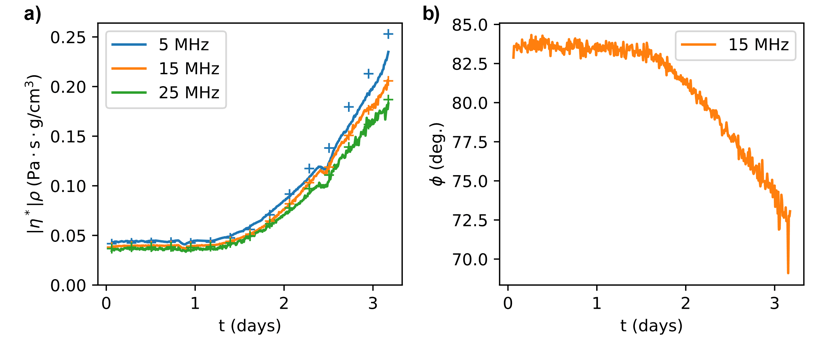 Figure 2: a) Complex viscosity for a bulk linseed oil QCM experiment. b) Viscoelastic phase angle for a bulk linseed oil QCM experiment.
Figure 2: a) Complex viscosity for a bulk linseed oil QCM experiment. b) Viscoelastic phase angle for a bulk linseed oil QCM experiment.
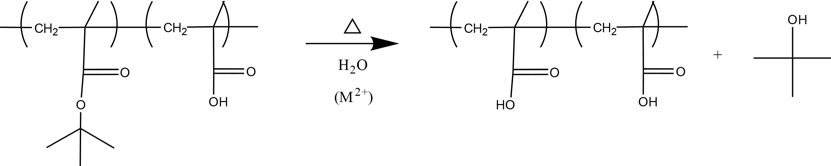 Figure 3: Reaction scheme for the hydrolysis of partially converted PtBMA. The M2+ indicates the metal cation, which will initially be Zn2+.
Figure 3: Reaction scheme for the hydrolysis of partially converted PtBMA. The M2+ indicates the metal cation, which will initially be Zn2+.
Topic 3: Nanoscale Studies of Zinc Soap Formation
Northwestern Postdoctoral Scholar: Stephanie Zaleski, Chemistry
Primary Advisors: Francesca Casadio (AIC), Katriene Keune (Rijksmuseum)
Key Outcome: A mechanistic description of metal soap formation in oil-based paints- a major form of deterioration of oil-based paint systems, linked to Topic 2 as a “real-life” example.
With this topic we aim to answer the following fundamental questions:
- What are the reaction kinetics of the formation and crystallization of Zn soaps in oil? What is the influence of environmental (temperature, humidity) and anthropic (solvents) parameters on such reaction pathways and kinetics?
- What happens at reaction boundaries between zinc oxide particles and the organic medium? Can we determine the structure (crystalline, amorphous, type of organic acid) and possible orientation of the newly formed zinc soaps at the nanoscale, from vibrational data?
Chemical and mechanical measurements at the micro/nano-scale will be performed with a combination of the quartz crystal microbalance (QCM, described in Topic 2) and tip enhanced Raman Spectroscopy (TERS). TERS is a scanning probe analog to Surface Enhanced Raman Spectroscopy (SERS), adding nanoscale spatial resolution to the high enhancement and single molecule sensitivity characteristics of SERS. The plasmonically active TERS scanning probe, typically 20-50 nm in diameter at the tip apex, allows taking TER spectra at points of interest on the scanned surface. In addition to the quartz crystal microbalance experiments undertaken in Topic 2, we will use FTIR-ATR to follow the effects of temperature, humidity and solvent exposure on reaction kinetics leading to crystallization of the Zn soaps (which are detrimental to paintings). We will also obtain cross sections by microtoming or using a focused ion beam (FIB), using tip-enhanced Raman scattering to probe nano-scale phases and reaction boundaries, as well as local environments in and around semi-crystalline domains of Zn soaps. From these experiments we will discern the type and orientation of fatty acid chains, and differences between semi-crystalline and amorphous zones of Zn soaps.
Current Focus: Significant results to date include the use of variable-temperature (VT) Raman spectroscopy to study the relationship between crystallinity and vibrational signature of synthesized zinc (Zn) carboxylates (Zn palmitate, Zn stearate). The degree of alkyl chain disorder is assessed by the peak intensity ratio of the asymmetric versus symmetric CH2 vibrational mode and the peak position of the symmetric CH2 mode. The peak intensities calculated from VT-Raman spectra (Figure 4) indicate that Zn soaps are crystalline solids at room temperature and disordered solids at temperatures between approximately 40°C up to the melting point (120-125°C), at which the Raman spectra is clearly indicative of the molten metal soap. The experimental results indicate the sensitivity of Raman spectroscopy to chain disorder in Zn carboxylates, which will be used as a guide to interpret acquired TERS spectral maps in model paint film systems. In addition to characterization of metal carboxylates by VT-Raman, model paint film samples consisting of ZnO and various drying oils (prepared at the Art Institute of Chicago) were sectioned by ultramicrotomy for analysis by TERS and scanning transmission electron microscopy (STEM). Sections ranging in thickness from 100 – 300 nanometers have been successfully prepared and characterized by atomic force microscopy and STEM. TERS measurements of the sectioned model paint films are currently underway.
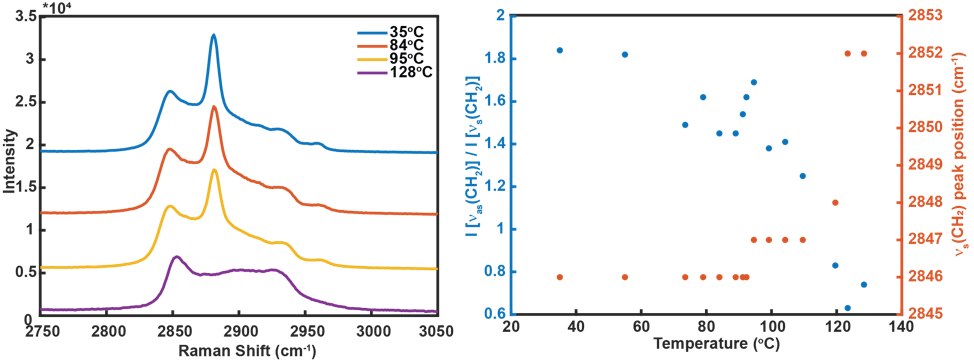 Figure 4: (left) Select VT-Raman spectra of Zinc stearate. (right) Temperature-dependence of the intensity ratio of the asymmetric and symmetric CH2 vibrational modes (blue dots) and position of the symmetric CH2 mode (red dots) of Zinc stearate, showing the increase in alkyl chain disorder with increasing temperature.
Figure 4: (left) Select VT-Raman spectra of Zinc stearate. (right) Temperature-dependence of the intensity ratio of the asymmetric and symmetric CH2 vibrational modes (blue dots) and position of the symmetric CH2 mode (red dots) of Zinc stearate, showing the increase in alkyl chain disorder with increasing temperature.
Topic 4: Photoactivity of TiO2- Based Formulations
Northwestern Student: Tom Schmitt, Materials Science and Engineering
Primary Advisors: Kimberly Gray (NU), Costanza Miliani (U. Perugia)
Key Outcome: A framework for understanding light-induced degradation of oil-based paints, using TiO2-pigmented systems as an experimental model.
In this topic we focus on paint degradation linked to the photochemistry of TiO2-filled oil paints, systems that are used as models for investigating photocatalytic effects. To understand the role of TiO2 pigments in the degradation of paints, it is crucial to define and explain the structure/photoactivity relationship in anatase, rutile and mixed-phases and in combination with sensitizer dyes. This will be effectively achieved by computational modeling simulations (by means of large scale DFT methods) on both the reference TiO2 phases and the mixed nanocomposites, mimicking the anatase/rutile interface. Dye degradation in sensitized TiO2 will be modeled by hypothesizing a degradation mechanism and computing the related reaction energetics. The dye-semiconductor hetero-interface will be characterized both in structural and electronic terms. The effect of TiO2 mixture with sensitizing organic dyes (photo-activity under visible light) will be modeled and an a priori screening will be carried out on a series of visible-absorbing organic dyes. The computational modeling will be supported with an experimental modeling carried out through accelerated aging experiments on model paints (made of TiO2 composites in combination with dyes and oily binding media) under quasi monochromatic lights to get the action spectra of various type of TiO2-based paint. Moreover, the effect of air constituents (such us SO2) as well as the role of moisture will be considered. A wide array of analytical methods will be exploited to characterize the alteration products at the micro-scale and their effect at the macro-scale on both optical and mechanical properties of the paint layers. This latter issue will be tackled in connection with topic 2 (characterization with the quartz crystal microbalance). The knowledge gained combining computational and experimental modeling will be profitably integrated and validated with analytical data at the micro (e.g. by micro-Raman, micro-FTIR, micro-UV-Vis fluorescence imaging at DISCO beamline in SOLEIL) and macro scale (by portable non-invasive methods belonging to the MOLAB facility) acquired on real paintings dated back to the early 20th century belonging to American and Italian modern art collections.
Current Focus: The main focus to date has been on paint degradation linked to the photochemistry of TiO2-filled oil paints, systems that are used as models for investigating photocatalytic effects. To understand the role of TiO2 pigments in the degradation of paints, it is crucial to define and explain the structure/photoactivity relationship in anatase, rutile and mixed-phases and in combination with sensitizer dyes. This will be effectively achieved by computational modeling simulations (by means of large scale DFT methods) on both the reference TiO2 phases and the mixed nanocomposites, mimicking the anatase/rutile interface. The computational modeling will be supported with an experimental modeling carried out through accelerated aging experiments on model paints (made of TiO2 composites in combination with dyes and oily binding media) under quasi monochromatic lights to get the action spectra of various type of TiO2-based paint.
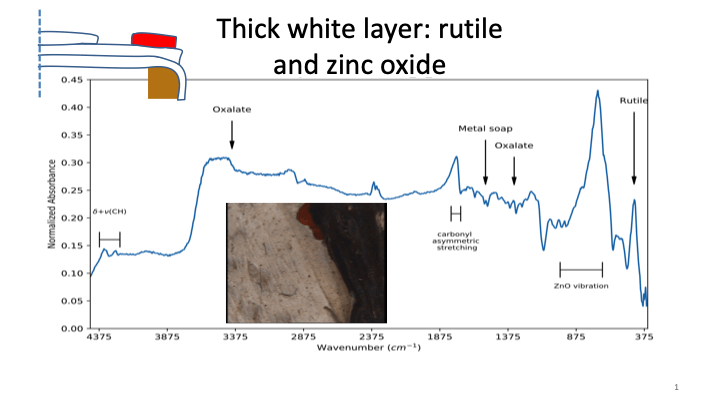
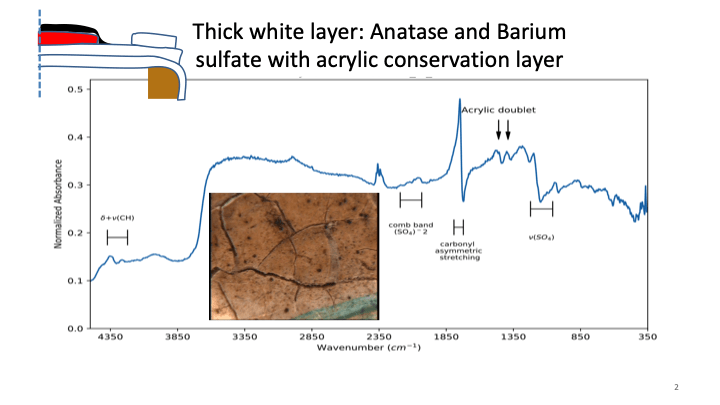 Figure 5: a) FTIR analysis of the rutile tianium dioxide containing paint confirms the presence of zinc oxide which was shown by FTIR spectroscopy to develop metal soaps. b) The area with anatase titanium dioxide paint was shown by FTIR to have a conservation treatment of acrylic coating.
Figure 5: a) FTIR analysis of the rutile tianium dioxide containing paint confirms the presence of zinc oxide which was shown by FTIR spectroscopy to develop metal soaps. b) The area with anatase titanium dioxide paint was shown by FTIR to have a conservation treatment of acrylic coating.
